Two dump trucks (240 tons each) in one of the 3 pits from Copper Mountain Mining Corp. (TSX-Symbol: CUM; market-cap.: $507 million) which company started producing high quality copper concentrates in July 2011 from its porphyry copper gold deposit some few km from Princeton in British Columbia (Canada). The 3 historic pits are set to be merged into 1 giant pit over the next next years giving birth to the „Superpit“ with a length of 7 km. The company has set up an offtake aggreement with Mitsubishi Materials Corp., wheareas it was agreed that the Japanese buy all of the concentrate output at the LME price on the day of shipment. The expected recovery rates are in the area of 89% copper, 66% gold, and 49% silver, wheareas no penalty fees for unwanted impurities must be paid. The Superpit is planned to get excavated by 480 tons of ore per day for at least 17 years, whereas the produced concentrate contains 28% Cu and a humidity of 8%. The resources stand at 5 billion pounds of contained copper (approx. 100 million pounds copper per year). The first concentrate shipment arrived in Onahama (Japan) on the 4th October – a total of 11,200 tons of concentrate containing 5.6 million pounds copper, 40,600 ounces silver and 2,470 ounces gold – around US$30 million worth. (Picture source: Copper Mountain Mining Corp. www.cumtn.com)

The newly built processing facility from Copper Mining Corp. near Princeton, BC (Canada)
Magmatic-hydrothermal Deposits
Magmatic-hydrothermal ores do not form like most other magmatic deposits by MAGMATIC SEGREGATION during the crystallization of the magma, but through the ASCENT thanks to hydrothermal activity by which means the ores are deposited in the earth crust in form of magmatic bodies. The typical types of magmatic hydrothermal deposits are on the one hand PORPHYRY and on the other hand VMS (volcanic-massive sulphide) deposits. Both represent the main supply of the globally mined copper and molybdenum.

The porphyry copper mine Bingham Canyon in Utah, USA
Furthermore, porphyry copper deposits gain an increasing significance in the mining of gold, whereas VMS deposits supply large quantities of zinc and lead on a worldwide basis. Porphyry deposits are the most important source for the metals copper, molybdenum and gold (more than 50% of the globally mined copper comes from porphyry systems). Additionally, significant quantities of other metals are mined from porphyry deposits, such as tin, silver, lead and zinc.
Large porphyry occurences are found for example in Germany, e.g. in the Thuringian Forrest, Saxony, Odenwald and in the Saale district. In northern Germany, scandinavian porphyries were found as glacial tills.

A rhyolite with numerous large crystals (white) within a fine- to coarse-grained matrix indicating that the magma at depth rose at a relatively late time towards surface
A PORPHYRY (i.e. a rock with a porphyry fabric/texture) is formed when at first the magma cools slowly at depth (forming large crystals that swim in the melt) and thereafter rises quickly towards surface (sometimes resulting in a volcanic eruption) cooling down the magma faster thus forming smaller crystals. Hence, overall a rock fabric has been produced consisting of large single crystals embedded within a microcrystalline groundmass/matrix. The large single crystals are typically macroscopic (i.e. can be seen by naked eye) and described as „inclusions“ having a size of a few mm up to numerous cm and an idiomorphic crystal form (i.e. completely shaped).
In general, one differentiates between quartz-rich and quartz-poor porphyries, whereas the first one hosts quartz crystals as inclusions being the reason for the naming of so-called QUARTZ-PORPHYRY, yet today the term RHYOLITE is used more commonly. However, even quartz-poor porphyries can include some or no quartz in the matrix. As the chemical composition can vary immensely, numerous descriptive terms are used, such as andesite, dacite, or trachyte.

A polished plate of a quartz-porphyry respectively a quartz-rich rhyolite from Thuringen, Germany
Generally, porphyry deposits are divided into the following types:
- Copper Porphyry Deposits
- Copper-Gold Porphyry Deposits
- Copper-Molybdenum Porphyry Deposits
- Molybdenum Porphyry Deposits



The porphyry copper mine Bingham Canyon in Utah, USA
Mineable porphyry deposits have an enormous size of some hundred million tons of extractable ores – with an average mineralization that can be as „low-grade“ as 0.2% to >1% copper, 0.005 to 0.03% molybdenum and/or 0.4 to 2 g/t gold.
In Canada the entire molybdenum and about half of the copper mining output originates from porphyry deposits, such as the Valley Copper Mine with 690 million tons averaging 0.41% copper; Island Copper with 345 million tons averaging 0.42% copper and 0.017% molybdenum; Brenda with 360 with 0.16% copper and 0.039% molybdenum; Mount Polley with 230 million tons averaging 0.25% copper and 0.34 g/t gold.
As porphyry deposits are generally quite low-grade, the mining must be accordingly cost-effective being the reason being typically mined via open-pits (if the erosion level allows).
Porphyry Copper Deposits
Geologists estimate that until 2,000 BC, copper grades in mining were around 15%. Some 3,500 years later, the mining grades were around 9%. Around 1800, the grades stood at 6-7% and mines successfully operated with such grades until the beginning of the 20th century.
 At the end of the 19th century, the Bingham Canyon Mine in Utah (USA) began with the mining of copper from veins that were mineralized with approx. 6% copper, whereafter the mine (with reserves of >290 million tons grading between 0.75-2.5%) changed into an open-pit – ushering in the new era of low-grade copper mining.
At the end of the 19th century, the Bingham Canyon Mine in Utah (USA) began with the mining of copper from veins that were mineralized with approx. 6% copper, whereafter the mine (with reserves of >290 million tons grading between 0.75-2.5%) changed into an open-pit – ushering in the new era of low-grade copper mining.
Bingham is the first porphyry copper mine in the world that profitably extracts copper from low-grade ores – and it is still one of the largest mining operations in the world (owners: Kennecott and Rio Tinto).
The >100 year mining project produced an open-pit with a depth of currently approx. 1.2 km and a length exceeding 4 km covering an area of around 8 km2. The open-pit is planned to continue until 2013, whereafter underground mining shall commence. Even today, Bingham is one of the most productive copper mines in the world. Until 2004, >17 million tons copper, 23 million ounces gold (715 tons), 190 million ounces silver (5,900 tons) and 850 million pounds (386,000 tons) molybdenum have been mined, whereas gold and silver are considered as by-products.
The value of all the metals mined up until today exceeds the combined output of Comstock Lode, Klondike and the Californian goldrush (solely the Chuquicamata Copper Mine in Chile excavated more copper than Bingham). The strongly increased molybdenum prices had the result that in 2005 more income was generated with molybdenum than with copper. In 2006, Bingham produced metals with a combined market value of US$1.8 billion. Some 1,400 workers organize with 64 dump trucks that 450,000 tons of rock are moved out
of the mine (one dump truck can transport approx. 240 tons). The mineralized rock is brought to conveyer belts that transport it 8 km to the processing facility. At the end of the 20th century, the deposit still had reserves of at least 1.7 billion tons averaging 0.71% copper.
It was the success of the Bingham Mine at the beginning of the last century that animated similar porphyr copper deposits, such as Globe-Miami (Arizona, USA), Eli (Nevada, USA), Santa Rita (New Mexico, USA) and Chuquicamata (Chile). Today, porphyry mines account for around half of all the newly mined copper worldwide. However, the success of mining low-grade copper ores went hand in hand with the technological advances in the metallurgical refinement and selective seperation of the copper sulphides („froth flotation“).


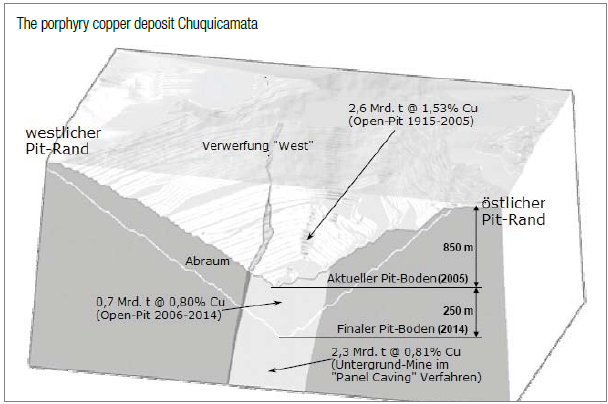
The porphyry copper deposit Chuquicamata in Chile
Chuquicamata (also known as Chuqui; 220 km north-east of Antofagasta in Chile) is the largest (known) copper deposit in the world. For many years, it was also the mine with the biggest yearly copper production, whereas in late 1990s it was overtopped by the Minera Escondida which was only discovered in 1978. In 2007, the mine produced some 1.5 million tons copper generating >US$10 billion (approx. 10% of the worldwide copper production respectively 26% of Chile`s output).
The reserves of the Escondida Mines still stand at around 35 million tons copper. Nonetheless and by large, Chuqui remains as the most productive mine as >30 million tons copper were extracted from 2.6 billion tons ore averaging 1.53% copper. Despite its almost 100 years of operation, Chuqui still hosts one of the biggest copper reserves worldwide. Today, the open-pit is approx. 1 km deep, 5 km long and 3 km wide. The neighboring smelter and electrolytic refinery are also among the largest processing facilities in the world.
Until 2014, some 700 million tons ore is expected to be lifted – at which time the open-pit shall reach a depth of approx. 1.1 km. Geological data suggests that another 2.3 billion tons ore averaging 0.81% copper lies between the 1.1. km deep pit bottom and 1.8 km depth. Models and plans are already designed to start mining via underground methods by 2020 to lift some 45 million tons ore per year until 2051.
In the beginning, only the high-grade copper mineralization in veins was mined having a copper grade of 10-15%. It was not until 1919 that the American engineer Bradley developed a technique to profitably extract from low-grade and oxidized ores. Thereafter, he engaged the well-established lawyer named Burrage whose engineers travelled to the deposit in order to examine it. As the results were considered as positive, the lawyer bought the mining concessions – yet, he did not have the appropriate amount of capital to develop itinto a mine. He enquired with the Guggenheim brothers who then as well sent a team to Chile estimating the reserves at 690 million tons averaging 2.58% copper. Guggenheim took over the mining concessions from the lawyer in exchange for $25 million in stocks of the newly founded Guggenheim-company Chilex.
The production started in 1915 with 4,500 tons copper in the first year increasing to 50,500 tons in 1920 and 136,000 tons by 1929 just before the Great Depression started shrinking demand drastically. Until 1951, the oxidized cap above the actual deposit was mined. Thereafter and thereunder, the secondary formed copper sulphides were mined which were formed by the leaching-out of the above oxidation zone.
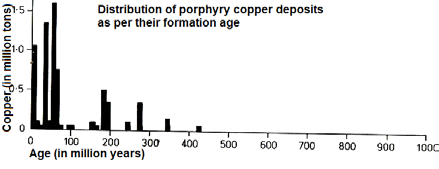
Age:
The main portion of all porphyry copper deposits was formed during the last 75 million years thus representing relatively young ore depositions. On the diagram can also be noted that these have a maximum age of a bit more than 400 million years. Only few porphyry copper deposits or occurences have been found with an age of more than 450 million years. One of the youngest is the Ok Tedi deposit in Papua Newguinea being approx. 1.2 million years of age. Currently, new porphyry copper deposits are formed somewhere in the world beneath active volcanic territory.
Size & Grades:
Typically, porphyry copper deposits are „low-grade“ yet being mined on a large scale via open-pits resulting in a processing of at least a few 100 million tons averaging mostly below 1% copper. In 1975, the 103 economic/mineable deposits in the world had an arithmetic average of 550 million tons averaging 0.6% copper. The table includes the world`s largest (economic) porphyry copper reserves exceeding 500 million tons at the end of the 20th century. Many of the largest deposits lie in the south-west of the USA besides South-America – all above the >10 billion tons large Chuquicamata Mine averaging 0.56% copper. Due to its large size, porphyry copper deposits are typically mined with open-pits. In the USA exist smaller deposits that are mined solely by underground methods. In stark contrast to this, much more porphyry molybdenum deposits are mined via underground. At the end of the last century, Canadian porphyry copper deposits averaged 150 million tons averaging 0.45% copper. The carbonatealkali deposit type (on average 206 million tons at 0.39% copper) had a higher tonnage with lower grades as the other alkali deposits (on average 49 million tons at 0.76% copper.

As the table informs, by products do not play an important role at all deposits. Typical byproducts include molybdenum, gold and silver, whereas oftentimes significant quantities of rhenium, tin and tungsten are found. The income from these by-products oftentimes plays a decisive role during feasibility studies. In general, porphyry copper deposits within the continental crust have a substantially higher gold content, whereas in areas of island arches significantly higher grades of molybdenum occur.
The Ok Tedi deposit in Papua Newguinea began production in 1984 with reserves of solely 350 million tons at 0.7% copper and 0.59 g/t gold. The top of the deposit had a ore zone with 34 million tons averaging 2.86 g/t gold. In the first 2 years, on average 12,000 tons ore was process per day containing 4.21 g/t gold on average. Without this initial income stream (respectively „start-up-capitalization“) from the gold recovery, the actual copper deposit beneath would not have been economic by itself.
In a similar way, Bougainville Copper Ltd. processed 130,000 tons ore per day from its Panuna deposit in Papua Newguinea in 1982 and extracted 170,000 t copper, 18 tons gold and 43 tons silver in that year alone generating an income of US$276 million, whereas gold had a total contribution of 47%.
The primary (hypogene) ore minerals are not only so-called disseminations (i.e. impregnated resp. finely included ore minerals within a porphyry rock) but mainly fillings of cracks resp. microscopic fractures and quartz veinlets that both contain variable amounts of pyrite, chalkopyrite, bornite and sometimes molybdenite.
Porphyry copper deposits are oftentimes titled and described as „disseminated“ as when looking at the large quantities of ore the distribution of ore minerals appears disseminate (i.e. finely included ore minerals within porphyry rock). Yet when looking at the mineralization from a closer perspective, one doubtlessly recognizes that the occurence of ore and the sulphidesfilled veins and veinlets are controlled by fractures in the rock. The geologists Beane and Titley estimate that >90% of the sulphide minerals either occur within or close to fractures. Those fractures can be as small as only detectable with a magnifying glass or microscope. It were these many big and small fractures that enabled the rock to be permeable for fluids enabling the mineralization process.
The diagram schematically shows the typical composition and distribution of ore minerals within intrusions, whereas it is notable that the more one goes from the core to the outer space the more fractures respectivel veins and veinlets occur (i.e. the red centre occurs completely disseminated, whereas the outer zones are characterized increasingly by fillings of rock fractures).
Generally, pyrite is the mostly abundant sulphide mineral in porphyry copper deposits. Magnetite has insignificant quantities within the red-yellow-striped centre, but has an abundancy in the red core besides halo #1 which covers the ore shells. The chalcosite content can increase from the ore shell towards the outside. Pyrrhotite and native copper sometimes occur within the yellow zone and within veins, however soley in small quantities or traces).
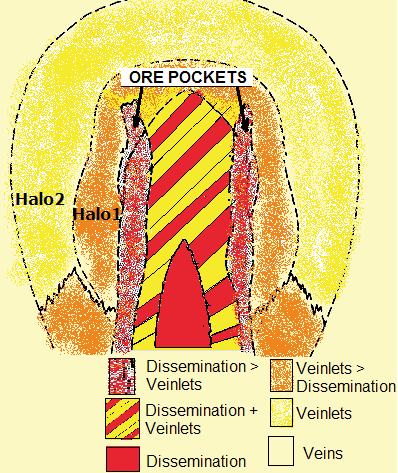
Yellow-red striped zone resp. centre of the intrusion: weakly to nonmineralized zone with small quantities of chalkopyrite, molybdenite and bornite besides <2% pyrite.
Ore shells: towards the outside, firstly mineralization of molybdenite and thereafter chalkopyrite, whereas the pyrite content increases continuously.
Halo 1 (orange; “pyrite shell”): much pyrite (10-15%) and magnetite with few chalkopyrite (0.1-3%) and molybdenite (in traces).
Halo 2 (yellow; peripheral zone): this outer mantle contains few pyrite but can include base metals within fractures and precious metals (especially gold and silver) within veins and veinlets.
Secondary (supergene) mineral enrichments not only represent a valuable mining zone but play an important role in the discovery of the same. This overlaying mantle includes minerals like chalcosite, djurleite and digenite, whereas native copper, cuprite and covellite can occur in significant quantities.
The formation of such (secondary enriched) oxidation and cementation zones is positively affected by hot and dry climates and the occurance of pyrite, whereas the host rock is chemically relatively inert. The shape of this secondary ore zone („supergene zone“) is dependent on the topography of the surface, the geometry and structure and hydrology of the intrusive body. These supergenely enriched zone are typically covered by a limonite-, hematite or jarosite containing rock which is (or shall be) considered at first when exploring for copper porphyries.
There are some few notable characteristics that can be supportive in that respect:
- Pyroxenes do not or rarely occur within copper porphyries.
- Hornblende + biotite occur richly.
- The extent of the rock fractures can be crucial as mineralized intrusives always occur within strongly fractured and fractionized rock strata.
- Brecciated zones are not rare and can occur within the intrusion itself or within the neighboring rocks. Breccias (apparently produced by hydrothermal activity) are oftentimes described as “pebble dykes”.
These typically have rounded fragments/clasts but also can be sharply angled „collapse/karst breccias“.
Alteration & Enrichment:
The hydrothermal effects not only result in alterations on the spot of the ore body, but usually as well in the neighboring zones. The alteration can change single minerals and as well complete rocks via metamorphosis. The extent of this alteration indicates that it was only possible through extensive permeability of the rock – hence, it is the fractures and cracks within the rock strata that support and control the alteration process.
The „selective alteration“ transforms 1 or 2 minerals into other minerals – in the case of porphyry copper deposits it is oftentimes hornblende and/or amphiboles that are transformed into (secondary) biotite and can occur in large quantities within the rock. Additionally, the biotite can then also be transformed into chlorite. The „pervasive alteration“ transforms complete rock types into different ones.
In the 1970s, Lowell and Guilbert developped a concept for a „typical porphyry deposit“. Their result is the so-called LOWELL-GUILBERT MODEL which showed that porphyry copper deposits have characterizable and recognizable hydrothermal alteration zones around the intrusion.
These alteration zones formed when overheated (aggressive) and mineral-rich hydrothermal fluids flowed through the fractured rock and reacted with the originally existing minerals (and partly transforming those into other kinds of minerals and/or completely forming new minerals).
The Lowell-Guilbert Model is still used by most geologists to interpret geochemical anomalies and to assist in the identification of drill targets – thus, the scientific findings and knowledge of this model can supply valuable insights for the exploration and development of such deposits.
The Lowell-Guilbert Model distinguishes 4 hydrothermally altered rock zones:
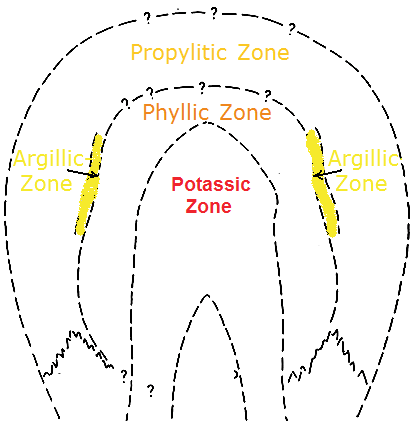 1. Potassic zone:
1. Potassic zone:
The fresh magma in the centre of the intrusion is mostly surrounded by a potassium-rich metasomatism-zone by the name potassic zone. The potassic alteration is the result of metasomatism that can be accompanied by the leaching out of calcium and natrium (from rocks that originally consisted of aluminium silicate minerals).
The typical minerals of this zone are biotite, orthoclase and quartz. These are typically accompanied by accessory albite, sericite, anhydrite and apatite, and as well chalkopyrite, bornite and pyrite. The pyrite always solely occurs in small quantities and is antithetic to bornite and magnetite.
The potassic alteration zone typically occurs within or close to the centre of the intrusion, whereas the neighboring rock can also be biotized. The primary formed feldspar minerals orthoclase and plagioclase, and as well other basic minerals are replaced/displaced by secondary biotite and orthoclase and/or chlorite. The inner part of this zone is mostly poor (or barren) in ore minerals.
2. Phyllic Zone:
This zone is also described as „quartz sericite zone“ and is the result of the leaching out of magnesium, natrium and calcium from aluminium silicate rocks. Potassium can be imported from the original rockforming feldspar mineral. The phyllic zone is characterized by quartz, sericite and pyrite, whereas the first 2 mentioned minerals typically completely replace the original rock-forming silicate minerals which leads to the result that the original rock structure and composition is completely destroyed resp. changed. The pyrite can grow resp. crystallize thanks to the original abundance of iron within basic minerals. The pyrite content can increase up to 10%, whereas chalkopyrite can occur but rarely exceeds 0.5% of the volume. Up to the next zone, the richness of clay minerals increases (so-called sericitization resp. advanced argillization). As the transformation of primary feldspars and biotite results in the release of significant quantities of silica, corresponding amounts of quartz are formed (so-called silicification). The copper-rich mineralization occurs oftentimes directly within this socalled „pyrite shell“ (within the transition area next to the potassic zone.
3. Argillic Zone:
This zone of intermediary argillization is characterized by newly formed clay minerals (especially kaolinite). Moderate argillic alteration result in montmorillonite, illite, chlorite and sometimes also kaolinite. Advanced argillic alteration leads to kaolinite with diaspor, quartz or amorphous chert/silica, andalusite and sometimes also korund. Pyrite is the main sulphide mineral, whereas also chalkopyrite and bornite can occur. However, pyrite is less abundant as within the phyllic zone and tend to occur in small veins/veinlets. The argillic zone is not formed in all porphyry copper deposits and can be completely absent.
4. Propylitic Zone:
This oftentimes extensively sized zone at the outer rim is always present and is characterized by chlorite, epidote and calcite (so-called propylitization). Although no copper mineralization occurs at or near the surface, this zone (which migrates to the neighboring rock) can be a valuable indication of the existence of a porphyry copper deposit. The potassium (which is formed by chloritization) occurs as (accessory) sericite. Other accessory minerals include: apatite, hematite/specularite, anhydrite and ankerite. Plagioclase also can appear unaltered. The occurence of sulphide minerals within this zone varies between none to small amounts of pyrite and occasionally economically significant concentrations of chalcopyrite. The mineralization occurs finely distributed/disseminated within the mother rock - mostly along smallest cracks, fissures and fractures, as well as in veinlets and veins. Hence, it is a so-called impregnation ore („disseminated ore“).
The term „stockworks“ is used when a lager and irregular network of veinlets is observed. Additionally, zones of fractured rock fragments can be found with angled/sharp and/or rounded edges can be found (so-called „breccia zone“).
The sulphidic ore enrichment (especially chalcopyrite and molybdenite) predominately occur in such fissures and cracks in form of mineralized veinlets or veins. Larger gaps between rocks are also filled with sulphide minerals or sulphide-rich quartz.
The high-grade mineralization occurs especially at those places where multiple independent vein- or joint-sets cross/intersect eachother. The ore hosting rock are mostly irregular to almost cylindrical intrusions and veins. Most frequently, these rocks are intermediate to acidic granites (with a decreasing content of silica, these include: granite, granodiorite, tonalite, quartz-monzonite and diorite; whereas additionally also an intermediate series exists from diorite and monzonite to syenite. It is assumed that such intrusions were always overlain by a volcanoe. In a much larger depth, porphyry magmatites typically change over to large plutonites with an uniform grained and mineralized composition.

Tectonic Settings
The above world map shows the largest porphyry deposits at the end of the 20th century and that most deposits are controlled by convergent plate boundaries. Furthermore, andesitic volcanism occured at these places. Virtually all porphyry copper deposits in the south-west of America were formed approx. 50 million years ago. The deposits of the Canadian Cordillera, in the Philippines and many in the Andes were also formed at that time.
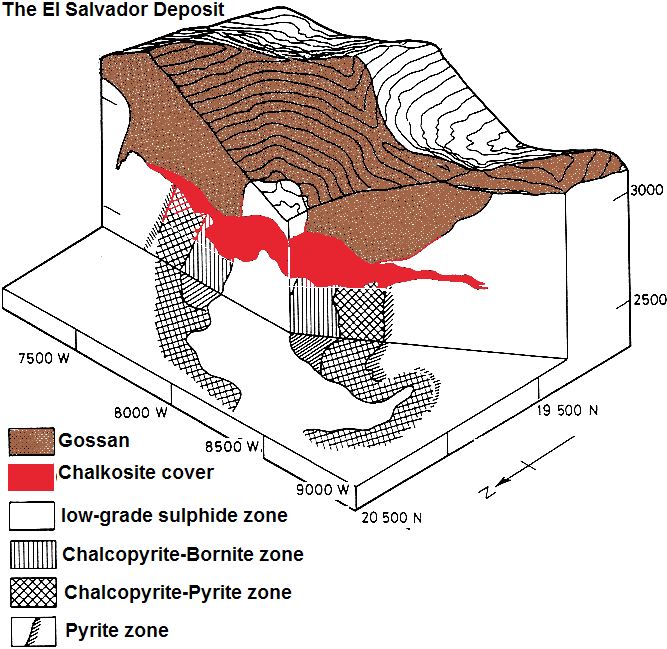
The Bingham Canyon deposit formed a bit earlier (some 37-41 million years ago) almost at the same time as the El Salvador deposit in Chile. The Andes (especially Chile and Peru) are home of deposits with an age between 4.3-5.9 million years which tend to occur along a linear-stretched belt some 100 km of today`s coastal boundaries and parallel to the plate boundaries.
Petrography
Porphyry copper deposits are bound to plutonic rocks (intrusions) with an intermediate to high silica (SiO2) content. The description of such deposits can appear misleading as the term „porphyry“ refers to the typical porphyry texture of the magmatic host rock and not to the texture of the ore mineralization.
The porphyry rock is characterized by largely formed single crystals within a fine-coarsed to glassy matrix. The intrusion – where the mineralization is the richest – is usually rather small (1-2 km diameter) and has a porphyry texture. Because of the partly high extent of rock and mineral alterations at the porphyry intrusions, it is mostly difficult to impossible to identify the original composition of the rock (however, generally calcium-carbonate-alkali type).
The typical ore hosting rocks are intrusions of the series granite (from adamellite and granodiorite to tonalite), whereas also diorite, syenite and quartz-monazites can be the mother rock. The composition of these rock types is mainly dependent on the location where the deposit was formed (granodiorites to quartz-diorites at island-arc areas and quartz-monazites to granodiorites at continental arcs).
It is notable that all porphyry copper deposit regions in the world are also home of intrusions that are poor or barren in ore mineralization eventhough having an equal or similar composition as their mineralized neighbors. Despite the advanced geophysical technologies it is not even today possible to clearly identify if a plutonite is richly mineralized or rather barren.
Formation:
Porphyry copper deposits are the result of emplacement (i.e. replacement by intrusions) of liquid magmas in highly permeable rock strata in relatively shallow depths within the earth`s crust. These intrusions and its fluid-phases produce the necessary heat and energy to partly produce the fractures itself and to produce the liquid convection currents through the rocks. These fluids are composed of vadose liquids and as well magmatic fluids.

The schematic diagram shows 2 models of hydrothermal systems for the formation of porphyry copper deposits, whereas orthomagnetic processes are displayed on the left and convective processes on the right.
It is assumed that portions of the liquid mother magma ascents from the magma chamber to approx. 1-2 km below the earth`s surface at which depths it gets stuck/freezes. In these depths, the crystallization of water-free minerals is enforced relatively quickly – thus, the remaining liquids and fluids (and other volatile matters) are increasingly enriched/concentrated within the remaining melt. This means that the mineralization phase of the mother/host rock oftentimes is connected to the youngest intrusion (as being the most differentiated/enriched/concentrated).
As the fluids are enriched increasingly, the vapor pressure increases simultaneously – until it exceeds the (neigboring) lithostatic (rock) pressure resulting in an abrupt degassing (gas release/venting) of the magma (hand in hand with a congruent increase in volume hence cracking and fracturing the neighboring rock strata). The closer the earth`s surface, the larger the volume increase of the gas-phase which partly explains the formation of breccia pipes (ascent channels, feeder conduits) within which the circulating fluids partly round and smooth the fractured rock edges.
Close to the surface, the magma cools relatively fast – hence producing small and uniform-coarsed crystalls in the matrix, whereas the earlier formed (larger) single crystals are enclosed (so-called porphyry texture). The inspection of the isotopes in gases and fluids that were enclosed within the rock showed that the assumption became a probability that a large portion of the hydrothermal fluids (incl. their high amounts of metals and sulfur.) These liquids cause the metasomatism of the potassic zone.
Due to the high temperature differences between the intrusion and the neigboring rock, vadose waters and crystal water in the neighboring rock is heated up und freed hence flowing into the hydrothermal fluids which leads to the formation of the outer alteration zones. Strong differences in terms of temperature, fluid-composition, salinity and pH-value exists in the transition area between such different (hydrothermal) systems which leads to the chemical deposition of the copper sulphides which are deposited in the mother rock.


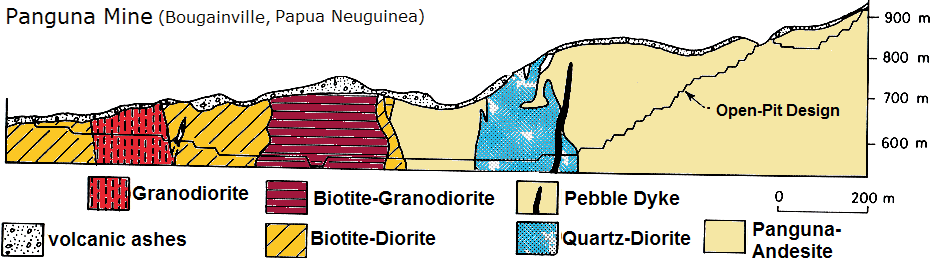
The Panguna Mine (see above pictures) mainly produced copper and gold from an open-pit between 1972-1989, whereas the mining stopped due to unrest, strikes and civil wars on the island. Although further reserves, infrastructue, a high willingness to work by the population and an income-hungry government (20% state ownership; 30% public; 50% Rio Tinto) exist today, no reopening of the mine occured yet. Due to the closure and the commencement of the civil war, the state income decreased by 20%. The costs of reopening are estimated at US$15 billion. Before the closure, the open-pit (which was the largest pit in the world at that time) produced some 180,000 tons copper and >400.000 ounces gold. The reserves are reported at 710 million tons ore averaging 0.4% copper and 0.47% g/t gold representing 10 million ounces gold. Thus, another 15 years of mining is possible.


Pyrite: FeS2
 The mineral pyrite, or iron pyrite, is an iron sulfide. This mineral‘s metallic luster and pale-to-normal, brass-yellow hue have earned it the nickname fool‘s gold because of its resemblance to gold. The color has also led to the nicknames brass, brazzle and Brazil, primarily used to refer to pyrite found in coal. Pyrite is the most common of the sulfide minerals. Pyrite is usually found associated with other sulfides or oxides in quartz veins, sedimentary rock, and metamorphic rock, as well as in coal beds, and as a replacement mineral in fossils. Despite being nicknamed fool‘s gold, pyrite is sometimes found in association with small quantities of gold. Gold and arsenic occur as a coupled substitution in the pyrite structure. In the Carlin, Nevada gold deposit, arsenian pyrite contains up to 0.37 wt% gold. Cattierite (CoS2) and Vaesite (NiS2) are similar in their structure and belong also to the pyrite group (so-called varieties).Bravoite is a nickel-cobalt bearing variety of pyrite, with >50% substitution of Ni2+ for Fe2+ within pyrite. Bravoite is not a formally recognised mineral. Chalcopyrite is brighter yellow with a greenish hue when wet and is softer (3.5–4 on Mohs‘ scale). Arsenopyrite is silver white and does not become more yellow when wet. Pyrite exposed to the atmosphere during mining and excavation reacts with oxygen and water to form sulfate, resulting in acid mine drainage. This acidity results from the action of Acidithiobacillus bacteria, which generate their energy by oxidizing ferrous iron (Fe2+) to ferric iron (Fe3+) using oxygen. The ferric iron in turn attacks the pyrite to produce ferrous iron and sulfate. The ferrous iron is then available for oxidation by the bacterium; this cycle continues until the pyrite is depleted. Iron pyrite oxidation is sufficiently exothermic that underground coal mines in high-sulfur coal seams have occasionally had serious problems with spontaneous combustion in the mined-out areas of the mine. The solution is to hermetically seal the mined-out areas to exclude oxygen.In modern coal mines, limestone dust is sprayed onto the exposed coal surfaces to reduce the hazard of dust explosions. This has the secondary benefit of neutralizing the acid released by pyrite oxidation and therefore slowing the oxidation cycle described above, thus reducing the likelihood of spontaneous combustion. In the long term, however, oxidation continues, and the hydrated sulfates formed may exert crystallization pressure that can expand cracks in the rock and lead eventually to roof fall. Building stone containing pyrite tends to stain brown as the pyrite oxidizes. This problem appears to be significantly worse if any marcasite is also present. The presence of pyrite in the aggregate used to make concrete can lead to severe deterioration as the pyrite oxidizes. In early 2009, problems with Chinese drywall imported into the United States after Hurricane Katrina were attributed to oxidation of pyrite. Text-Source: Wikipedia. Pciture: 2.8 x 2.7 x 2.4 cm large pyrite cube; Locality: Navajún, Rioja, Spain; Source: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0, www.mindat.org/photo-290488.html
The mineral pyrite, or iron pyrite, is an iron sulfide. This mineral‘s metallic luster and pale-to-normal, brass-yellow hue have earned it the nickname fool‘s gold because of its resemblance to gold. The color has also led to the nicknames brass, brazzle and Brazil, primarily used to refer to pyrite found in coal. Pyrite is the most common of the sulfide minerals. Pyrite is usually found associated with other sulfides or oxides in quartz veins, sedimentary rock, and metamorphic rock, as well as in coal beds, and as a replacement mineral in fossils. Despite being nicknamed fool‘s gold, pyrite is sometimes found in association with small quantities of gold. Gold and arsenic occur as a coupled substitution in the pyrite structure. In the Carlin, Nevada gold deposit, arsenian pyrite contains up to 0.37 wt% gold. Cattierite (CoS2) and Vaesite (NiS2) are similar in their structure and belong also to the pyrite group (so-called varieties).Bravoite is a nickel-cobalt bearing variety of pyrite, with >50% substitution of Ni2+ for Fe2+ within pyrite. Bravoite is not a formally recognised mineral. Chalcopyrite is brighter yellow with a greenish hue when wet and is softer (3.5–4 on Mohs‘ scale). Arsenopyrite is silver white and does not become more yellow when wet. Pyrite exposed to the atmosphere during mining and excavation reacts with oxygen and water to form sulfate, resulting in acid mine drainage. This acidity results from the action of Acidithiobacillus bacteria, which generate their energy by oxidizing ferrous iron (Fe2+) to ferric iron (Fe3+) using oxygen. The ferric iron in turn attacks the pyrite to produce ferrous iron and sulfate. The ferrous iron is then available for oxidation by the bacterium; this cycle continues until the pyrite is depleted. Iron pyrite oxidation is sufficiently exothermic that underground coal mines in high-sulfur coal seams have occasionally had serious problems with spontaneous combustion in the mined-out areas of the mine. The solution is to hermetically seal the mined-out areas to exclude oxygen.In modern coal mines, limestone dust is sprayed onto the exposed coal surfaces to reduce the hazard of dust explosions. This has the secondary benefit of neutralizing the acid released by pyrite oxidation and therefore slowing the oxidation cycle described above, thus reducing the likelihood of spontaneous combustion. In the long term, however, oxidation continues, and the hydrated sulfates formed may exert crystallization pressure that can expand cracks in the rock and lead eventually to roof fall. Building stone containing pyrite tends to stain brown as the pyrite oxidizes. This problem appears to be significantly worse if any marcasite is also present. The presence of pyrite in the aggregate used to make concrete can lead to severe deterioration as the pyrite oxidizes. In early 2009, problems with Chinese drywall imported into the United States after Hurricane Katrina were attributed to oxidation of pyrite. Text-Source: Wikipedia. Pciture: 2.8 x 2.7 x 2.4 cm large pyrite cube; Locality: Navajún, Rioja, Spain; Source: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0, www.mindat.org/photo-290488.html
Chalcopyrite: CuFeS2
 Chalcopyrite is a copper iron sulfide mineral that crystallizes in the tetragonal system. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green tinged black. On exposure to air, chalcopyrite oxidises to a variety of oxides, hydroxides and sulfates. Associated copper minerals include the sulfides bornite (Cu5FeS4), chalcocite (Cu2S), covellite (CuS), digenite (Cu9S5); carbonates such as malachite and azurite, and rarely oxides such as cuprite (Cu2O). Chalcopyrite is rarely found in association with native copper. Chalcopyrite is often confused with pyrite, although the latter has a cubic and not a tetragonal crystal system. Further, chalcopyrite is often massive, rarely crystalline, and less brittle. Chalcopyrite is also a darker yellow in color, with a greenish tinge and diagnostic greasy luster. Natural chalcopyrite has no solid solution series with any other sulfide minerals. There is limited substitution of Zn with Cu despite chalcopyrite having the same crystal structure as sphalerite. However, it is often contaminated by a variety of other trace elements such as Co, Ni, Mn, Zn and Sn substituting for Cu and Fe. Se, Fe and As substitute for sulfur, and trace amounts of Ag, Au, Pt, Pd, Pb, V, Cr, In, Al and Sb are reported. It is likely many of these elements are present in finely intergrown minerals within the chalcopyrite crystal, for instance lamellae of arsenopyrite representing As, molybdenite representing Mo, etc. Due to its color and high copper content, chalcopyrite has often been referred to as „yellow copper“ or „fool‘s gold“. Chalcopyrite is present with many ore bearing environments via a variety of ore forming processes. Chalcopyrite is present in volcanogenic massive sulfide ore deposits and sedimentary exhalative deposits, formed by deposition of copper during hydrothermal circulation. Chalcopyrite is concentrated in this environment via fluid transport. Porphyry copper ore deposits are formed by concentration of copper within a granite stock during the ascent and crystallisation of a magma. Chalcopyrite in this environment is produced by concentration within a magma system. Chalcopyrite is an accessory mineral in Kambalda type komatiitic nickel ore deposits, formed from an immiscible sulfide liquid in sulfur-saturated ultramafic lavas. In this environment chalcopyrite is formed by a sulfide liquid stripping copper from an immiscible silicate liquid. Chalcopyrite is the most important copper ore. Chalcopyrite ore occurs in a variety of ore types, from huge masses as at Timmins, Ontario, to irregular veins and disseminations associated with granitic to dioritic intrusives as in the porphyry copper deposits of Broken Hill, the American cordillera and the Andes. The largest deposit of nearly pure chalcopyrite ever discovered in Canada was at the southern end of the Temagami greenstone belt where Copperfields Mine extracted the high-grade copper. Chalcopyrite is present in the supergiant Olympic Dam Cu-Au-U deposit in South Australia. Chalcopyrite may also be found in coal seams associated with pyrite nodules, and as disseminations in carbonate sedimentary rocks. Crystallographically the structure of chalcopyrite is closely related to that of zinc blende ZnS (sphalerite). The unit cell is twice as large, reflecting an alternation of Cu+ and Fe3+ ions replacing Zn2+ ions in adjacent cells. In contrast to the pyrite structure chalcopyrite has single S2- sulfide anions rather than disulfide pairs. Another difference is that the iron cation is not diamagnetic low spin Fe(II) as in pyrite. Text-Source: Wikipedia. Picture: gold-colored chalcopyrite on redish rhodochrosite besides stilbite and sphalerite; Size: 9.3 x 6.6 x 2.5 cm; Locality: Starnitsa deposit, Rhodope Mountains, Smolyan Oblast, Bulgaria; Source: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0, www.mindat.org/photo-242352.html
Chalcopyrite is a copper iron sulfide mineral that crystallizes in the tetragonal system. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green tinged black. On exposure to air, chalcopyrite oxidises to a variety of oxides, hydroxides and sulfates. Associated copper minerals include the sulfides bornite (Cu5FeS4), chalcocite (Cu2S), covellite (CuS), digenite (Cu9S5); carbonates such as malachite and azurite, and rarely oxides such as cuprite (Cu2O). Chalcopyrite is rarely found in association with native copper. Chalcopyrite is often confused with pyrite, although the latter has a cubic and not a tetragonal crystal system. Further, chalcopyrite is often massive, rarely crystalline, and less brittle. Chalcopyrite is also a darker yellow in color, with a greenish tinge and diagnostic greasy luster. Natural chalcopyrite has no solid solution series with any other sulfide minerals. There is limited substitution of Zn with Cu despite chalcopyrite having the same crystal structure as sphalerite. However, it is often contaminated by a variety of other trace elements such as Co, Ni, Mn, Zn and Sn substituting for Cu and Fe. Se, Fe and As substitute for sulfur, and trace amounts of Ag, Au, Pt, Pd, Pb, V, Cr, In, Al and Sb are reported. It is likely many of these elements are present in finely intergrown minerals within the chalcopyrite crystal, for instance lamellae of arsenopyrite representing As, molybdenite representing Mo, etc. Due to its color and high copper content, chalcopyrite has often been referred to as „yellow copper“ or „fool‘s gold“. Chalcopyrite is present with many ore bearing environments via a variety of ore forming processes. Chalcopyrite is present in volcanogenic massive sulfide ore deposits and sedimentary exhalative deposits, formed by deposition of copper during hydrothermal circulation. Chalcopyrite is concentrated in this environment via fluid transport. Porphyry copper ore deposits are formed by concentration of copper within a granite stock during the ascent and crystallisation of a magma. Chalcopyrite in this environment is produced by concentration within a magma system. Chalcopyrite is an accessory mineral in Kambalda type komatiitic nickel ore deposits, formed from an immiscible sulfide liquid in sulfur-saturated ultramafic lavas. In this environment chalcopyrite is formed by a sulfide liquid stripping copper from an immiscible silicate liquid. Chalcopyrite is the most important copper ore. Chalcopyrite ore occurs in a variety of ore types, from huge masses as at Timmins, Ontario, to irregular veins and disseminations associated with granitic to dioritic intrusives as in the porphyry copper deposits of Broken Hill, the American cordillera and the Andes. The largest deposit of nearly pure chalcopyrite ever discovered in Canada was at the southern end of the Temagami greenstone belt where Copperfields Mine extracted the high-grade copper. Chalcopyrite is present in the supergiant Olympic Dam Cu-Au-U deposit in South Australia. Chalcopyrite may also be found in coal seams associated with pyrite nodules, and as disseminations in carbonate sedimentary rocks. Crystallographically the structure of chalcopyrite is closely related to that of zinc blende ZnS (sphalerite). The unit cell is twice as large, reflecting an alternation of Cu+ and Fe3+ ions replacing Zn2+ ions in adjacent cells. In contrast to the pyrite structure chalcopyrite has single S2- sulfide anions rather than disulfide pairs. Another difference is that the iron cation is not diamagnetic low spin Fe(II) as in pyrite. Text-Source: Wikipedia. Picture: gold-colored chalcopyrite on redish rhodochrosite besides stilbite and sphalerite; Size: 9.3 x 6.6 x 2.5 cm; Locality: Starnitsa deposit, Rhodope Mountains, Smolyan Oblast, Bulgaria; Source: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0, www.mindat.org/photo-242352.html
Bornite: Cu5FeS4
 It was first described in 1725 for an occurrence in the Krušné Hory Mountains (Erzgebirge), Karlovy Vary Region, Bohemia in what is now the Czech Republic. It was named in 1845 for Austrian mineralogist Ignaz von Born (1742–1791). Bornite is a sulfide mineral that crystallizes in the orthorhombic system (pseudo-cubic). Bornite has a brown to copper-red color on fresh surfaces that tarnishes to various iridescent shades of blue to purple in places. Its striking iridescence gives it the nickname peacock copper or peacock ore. Bornite is an important copper ore mineral and occurs widely in porphyry copper deposits along with the more common chalcopyrite. Chalcopyrite and bornite are both typically replaced by chalcocite and covellite in the supergene enrichment zone of copper deposits. Bornite is also found as disseminations in mafic igneous rocks, in contact metamorphic skarn deposits, in pegmatites and in sedimentary cupriferous shales. It is important as an ore for its copper content of about 63 percent by mass. It occurs globally in copper ores with notable crystal localities in Butte, Montana and at Bristol, Connecticut in the U. S. It is also collected from the Carn Brea mine, Illogan, and elsewhere in Cornwall, England. Large crystals are found from the Frossnitz Alps, eastern Tirol, Austria; the Mangula mine, Lomagundi district, Zimbabwe; from the NÂ’ouva mine, Talate, Morocco, the West Coast of Tasmania and in Dzhezkazgan, Kazakhstan. Text-Source: Wikipedia. Picture: greenish-oxidized bornite crystals from the Dzhezkazgan deposit (Zhezkazgan Mine) near Schesqasghan in Kasachstan; Viewing field 7 mm; Locality: Dzhezkazgan Mine (Zhezkazgan Mine), Schesqasghan, Zhezqazghan Oblysy , Kasachstan; Source: Leon Hupperichs, www.mindat.org/photo-105006.html
It was first described in 1725 for an occurrence in the Krušné Hory Mountains (Erzgebirge), Karlovy Vary Region, Bohemia in what is now the Czech Republic. It was named in 1845 for Austrian mineralogist Ignaz von Born (1742–1791). Bornite is a sulfide mineral that crystallizes in the orthorhombic system (pseudo-cubic). Bornite has a brown to copper-red color on fresh surfaces that tarnishes to various iridescent shades of blue to purple in places. Its striking iridescence gives it the nickname peacock copper or peacock ore. Bornite is an important copper ore mineral and occurs widely in porphyry copper deposits along with the more common chalcopyrite. Chalcopyrite and bornite are both typically replaced by chalcocite and covellite in the supergene enrichment zone of copper deposits. Bornite is also found as disseminations in mafic igneous rocks, in contact metamorphic skarn deposits, in pegmatites and in sedimentary cupriferous shales. It is important as an ore for its copper content of about 63 percent by mass. It occurs globally in copper ores with notable crystal localities in Butte, Montana and at Bristol, Connecticut in the U. S. It is also collected from the Carn Brea mine, Illogan, and elsewhere in Cornwall, England. Large crystals are found from the Frossnitz Alps, eastern Tirol, Austria; the Mangula mine, Lomagundi district, Zimbabwe; from the NÂ’ouva mine, Talate, Morocco, the West Coast of Tasmania and in Dzhezkazgan, Kazakhstan. Text-Source: Wikipedia. Picture: greenish-oxidized bornite crystals from the Dzhezkazgan deposit (Zhezkazgan Mine) near Schesqasghan in Kasachstan; Viewing field 7 mm; Locality: Dzhezkazgan Mine (Zhezkazgan Mine), Schesqasghan, Zhezqazghan Oblysy , Kasachstan; Source: Leon Hupperichs, www.mindat.org/photo-105006.html
Chalcosite: Cu2S
 Chalcocite, copper(I) sulfide is an important copper ore mineral. It is opaque, being colored dark-gray to black with a metallic luster. It has a hardness of 2½ - 3. It is a sulfide with an orthorhombic crystal system. The term chalcocite comes from the alteration of the obsolete name chalcosine, from the Greek khalkos, meaning copper. It is also known as redruthite, vitreous copper and copper-glance. Chalcocite is sometimes found as a primary vein mineral in hydrothermal veins. However, most chalcocite occurs in the supergene enriched environment below the oxidation zone of copper deposits as a result of the leaching of copper from the oxidized minerals. It is also often found in sedimentary rocks. It has been mined for centuries and is one of the most profitable copper ores. The reasons for this is its high copper content (67% atomic ratio and nearly 80% by weight) and the ease at which copper can be separated from sulfur. Since chalcocite is a secondary mineral that forms from the alteration of other minerals, it has been known to form pseudomorphs of many different minerals. A pseudomorph is a mineral that has replaced another mineral atom by atom, but it leaves the original mineral‘s crystal shape intact. Chalcocite has been known to form pseudomorphs of the minerals bornite, covellite, chalcopyrite, pyrite, enargite, millerite, galena and sphalerite. Text Source: Wiklipedia; Picture: typically greyish-black chalcocite crystallizations with metal lustre; Source: United States Geological Survey and Mineral Information Institute, USA.
Chalcocite, copper(I) sulfide is an important copper ore mineral. It is opaque, being colored dark-gray to black with a metallic luster. It has a hardness of 2½ - 3. It is a sulfide with an orthorhombic crystal system. The term chalcocite comes from the alteration of the obsolete name chalcosine, from the Greek khalkos, meaning copper. It is also known as redruthite, vitreous copper and copper-glance. Chalcocite is sometimes found as a primary vein mineral in hydrothermal veins. However, most chalcocite occurs in the supergene enriched environment below the oxidation zone of copper deposits as a result of the leaching of copper from the oxidized minerals. It is also often found in sedimentary rocks. It has been mined for centuries and is one of the most profitable copper ores. The reasons for this is its high copper content (67% atomic ratio and nearly 80% by weight) and the ease at which copper can be separated from sulfur. Since chalcocite is a secondary mineral that forms from the alteration of other minerals, it has been known to form pseudomorphs of many different minerals. A pseudomorph is a mineral that has replaced another mineral atom by atom, but it leaves the original mineral‘s crystal shape intact. Chalcocite has been known to form pseudomorphs of the minerals bornite, covellite, chalcopyrite, pyrite, enargite, millerite, galena and sphalerite. Text Source: Wiklipedia; Picture: typically greyish-black chalcocite crystallizations with metal lustre; Source: United States Geological Survey and Mineral Information Institute, USA.
Pyrrhotite: Fe7S8
 The name pyrrhotite is derived from Greek pyrrhos, flame-colored. Pyrrhotite is an unusual iron sulfide mineral with a variable iron content: Fe(1-x)S (x = 0 to 0.2). The FeS endmember is known as troilite. Pyrrhotite is also called magnetic pyrite because the color is similar to pyrite and it is weakly magnetic. The magnetism increases as the iron content decreases, and the troilite is non-magnetic. The ideal FeS lattice, such as that of troilite, is non-magnetic. The ferromagnetism which is widely observed in pyrrhotite is therefore attributed to the presence of relatively large concentrations of iron vacancies (up to 20%) in the crystal structure. Vacancies lower the crystal symmetry. Therefore, monoclinic forms of pyrrhotite are in general more defectrich than the more symmetrical hexagonal forms, and thus are more magnetic. Upon heating to 320 °C, pyrrhotite loses its magnetism, but also starts decomposing to magnetite. The saturation magnetization of pyrrhotite is 0.12 tesla. Pyrrhotite is a rather common trace constituent of mafic igneous rocks especially norites. It occurs as segregation deposits in layered intrusions associated with pentlandite, chalcopyrite and other sulfides. It is an important constituent of the Sudbury intrusion where it occurs in masses associated with copper and nickel mineralisation. It also occurs in pegmatites and in contact metamorphic zones. Pyrrhotite is often accompanied by pyrite, marcasite and magnetite. Pyrrhotite does not have specific applications. It is mined primarily because it is associated with pentlandite, sulfide mineral that can contain significant amounts of nickel and cobalt. Text Source: Wikipedia; Picture: pyrrhotite crystallization (with oxidation stains) from the Trep Valley, Kosovska Mitrovica, Kosovo; Size: 4.8 x 4.1 x 3.4 cm; Source: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0, www.mindat.org/photo-195241.html
The name pyrrhotite is derived from Greek pyrrhos, flame-colored. Pyrrhotite is an unusual iron sulfide mineral with a variable iron content: Fe(1-x)S (x = 0 to 0.2). The FeS endmember is known as troilite. Pyrrhotite is also called magnetic pyrite because the color is similar to pyrite and it is weakly magnetic. The magnetism increases as the iron content decreases, and the troilite is non-magnetic. The ideal FeS lattice, such as that of troilite, is non-magnetic. The ferromagnetism which is widely observed in pyrrhotite is therefore attributed to the presence of relatively large concentrations of iron vacancies (up to 20%) in the crystal structure. Vacancies lower the crystal symmetry. Therefore, monoclinic forms of pyrrhotite are in general more defectrich than the more symmetrical hexagonal forms, and thus are more magnetic. Upon heating to 320 °C, pyrrhotite loses its magnetism, but also starts decomposing to magnetite. The saturation magnetization of pyrrhotite is 0.12 tesla. Pyrrhotite is a rather common trace constituent of mafic igneous rocks especially norites. It occurs as segregation deposits in layered intrusions associated with pentlandite, chalcopyrite and other sulfides. It is an important constituent of the Sudbury intrusion where it occurs in masses associated with copper and nickel mineralisation. It also occurs in pegmatites and in contact metamorphic zones. Pyrrhotite is often accompanied by pyrite, marcasite and magnetite. Pyrrhotite does not have specific applications. It is mined primarily because it is associated with pentlandite, sulfide mineral that can contain significant amounts of nickel and cobalt. Text Source: Wikipedia; Picture: pyrrhotite crystallization (with oxidation stains) from the Trep Valley, Kosovska Mitrovica, Kosovo; Size: 4.8 x 4.1 x 3.4 cm; Source: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0, www.mindat.org/photo-195241.html
Molybdenite: MoS2
 Molybdenite is a mineral of molybdenum disulfide. Similar in appearance and feel to graphite, molybdenite has a lubricating effect that is a consequence of its layered structure. The atomic structure consists of a sheet of molybdenum atoms sandwiched between sheets of sulfur atoms. The Mo-S bonds are strong, but the interaction between the sulfur atoms at the top and bottom of separate sandwichlike tri-layers is weak, resulting in easy slippage as well as cleavage planes. Molybdenite crystallizes in the hexagonal crystal system as the common polytype 2H and also in the trigonal system as the 3R polytype. Molybdenite occurs in high temperature hydrothermal ore deposits. Its associated minerals include pyrite, chalcopyrite, quartz, anhydrite, fluorite, and scheelite. Important deposits include the disseminated porphyry molybdenum deposits at Questa, New Mexico and the Henderson and Climax mines in Colorado. Molybdenite also occurs in porphyry copper deposits of Arizona, Utah, and Mexico. The element rhenium is always present in molybdenite as a substitute for molybdenum, usually in the parts per million (ppm) range, but often up to 1–2%. High rhenium content results in a structural variety detectable by X-ray diffraction techniques. Molybdenite ores are essentially the only source for rhenium. The presence of the radioactive isotope rhenium-187 and its daughter isotope osmium-187 provides a useful geochronologic dating technique. Molybdenite flakes are a direct bandgap semiconductor with good charge mobility and can be used to create small or low-voltage transistors possibly easier than graphene. Text Source: Wikipedia; Picture: molybdenite on quartz from the Molly Hill Mine, Quebec, Canada; Source: John Chapman.
Molybdenite is a mineral of molybdenum disulfide. Similar in appearance and feel to graphite, molybdenite has a lubricating effect that is a consequence of its layered structure. The atomic structure consists of a sheet of molybdenum atoms sandwiched between sheets of sulfur atoms. The Mo-S bonds are strong, but the interaction between the sulfur atoms at the top and bottom of separate sandwichlike tri-layers is weak, resulting in easy slippage as well as cleavage planes. Molybdenite crystallizes in the hexagonal crystal system as the common polytype 2H and also in the trigonal system as the 3R polytype. Molybdenite occurs in high temperature hydrothermal ore deposits. Its associated minerals include pyrite, chalcopyrite, quartz, anhydrite, fluorite, and scheelite. Important deposits include the disseminated porphyry molybdenum deposits at Questa, New Mexico and the Henderson and Climax mines in Colorado. Molybdenite also occurs in porphyry copper deposits of Arizona, Utah, and Mexico. The element rhenium is always present in molybdenite as a substitute for molybdenum, usually in the parts per million (ppm) range, but often up to 1–2%. High rhenium content results in a structural variety detectable by X-ray diffraction techniques. Molybdenite ores are essentially the only source for rhenium. The presence of the radioactive isotope rhenium-187 and its daughter isotope osmium-187 provides a useful geochronologic dating technique. Molybdenite flakes are a direct bandgap semiconductor with good charge mobility and can be used to create small or low-voltage transistors possibly easier than graphene. Text Source: Wikipedia; Picture: molybdenite on quartz from the Molly Hill Mine, Quebec, Canada; Source: John Chapman.
Magnetite: Fe3O4
 Magnetite is a ferrimagnetic mineral, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron(II,III) oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part wüstite (FeO) and one part hematite (Fe2O3). This refers to the different oxidation states of the iron in one structure, not a solid solution. The Curie temperature of magnetite is 858 K (585 °C; 1,085 °F). It is black or brownish-black with a metallic luster, has a Mohs hardness of 5–6 and a black streak. Magnetite is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, and this was how ancient people first noticed the property of magnetism. Lodestones were used as an early form of magnetic compass. Magnetite typically carries the dominant magnetic signature in rocks, and so it has been a critical tool in paleomagnetism, a science important in discovering and understanding plate tectonics and as historic data for magnetohydrodynamics and other scientific fields. The relationships between magnetite and other iron-rich oxide minerals such as ilmenite, hematite, and ulvospinel have been much studied, as the complicated reactions between these minerals and oxygen influence how and when magnetite preserves records of the Earth‘s magnetic field. Magnetite has been very important in understanding the conditions under which rocks form. Magnetite reacts with oxygen to produce hematite, and the mineral pair forms a buffer that can control oxygen fugacity. Commonly, igneous rocks contain grains of two solid solutions, one between magnetite and ulvospinel and the other between ilmenite and hematite. Compositions of the mineral pairs are used to calculate how oxidizing was the magma (i.e., the oxygen fugacity of the magma): a range of oxidizing conditions are found in magmas and the oxidation state helps to determine how the magmas might evolve by fractional crystallization. Small grains of magnetite occur in almost all igneous and metamorphic rocks. Magnetite also occurs in many sedimentary rocks, including banded iron formations. In many igneous rocks, magnetite-rich and ilmenite-rich grains occur that precipitated together from magma. Magnetite also is produced from peridotites and dunites by serpentinization. Magnetite is sometimes found in large quantities in beach sand. Such black sands (mineral sands or iron sands) are found in various places, such as California and the west coast of New Zealand. The magnetite is carried to the beach via rivers from erosion and is concentrated via wave action and currents. Huge deposits have been found in banded iron formations. These sedimentary rocks have been used to infer changes in the oxygen content of the atmosphere of the Earth. Large deposits of magnetite are also found in the Atacama region of Chile, Valentines region of Uruguay, Kiruna, Sweden, the Pilbara, Midwest and Northern Goldfields regions in Western Australia, New South Wales in the Tallawang Region, and in the Adirondack region of New York in the United States. Deposits are also found in Norway, Germany, Italy, Switzerland, South Africa, India, Mexico, and in Oregon, New Jersey, Pennsylvania, North Carolina, Virginia, New Mexico, Utah, and Colorado in the United States. In 2005, an exploration company, Cardero Resources, discovered a vast deposit of magnetite-bearing sand dunes in Peru. The dune field covers 250 square kilometers (100 sq mi), with the highest dune at over 2,000 meters (6,560 ft) above the desert floor. The sand contains 10% magnetite. Crystals of magnetite have been found in some bacteria (e.g., Magnetospirillum magnetotacticum) and in the brains of bees, termites, fish, some birds (e.g., the pigeon) and humans. These crystals are thought to be involved in magnetoreception, the ability to sense the polarity or the inclination of the Earth‘s magnetic field, and to be involved in navigation. Also, chitons have teeth made of magnetite on their radula, making them unique among animals. This means they have an exceptionally abrasive tongue with which to scrape food from rocks. The study of biomagnetism began with the discoveries of Caltech paleoecologist Heinz Lowenstam in the 1960s. Because of its stability at high temperatures, it is used for coating industrial water tube steam boilers. The magnetite layer is formed after a chemical treatment (e.g. by using hydrazine). Magnetite is also used as a catalyst for various industrial chemical processes, such as: Fischer-Tropsch process, the Haber-Bosch process and the water gas shift reaction. Text Source: Wikipedia; Picture: magnetite on chalcopyrite (gold-colored); Size: 7 x 6 x 4 cm; Locality: Aggeneys, Nordkap, South-Africa; Source: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0, www.mindat.org/photo-251632.html
Magnetite is a ferrimagnetic mineral, one of several iron oxides and a member of the spinel group. The chemical IUPAC name is iron(II,III) oxide and the common chemical name is ferrous-ferric oxide. The formula for magnetite may also be written as FeO·Fe2O3, which is one part wüstite (FeO) and one part hematite (Fe2O3). This refers to the different oxidation states of the iron in one structure, not a solid solution. The Curie temperature of magnetite is 858 K (585 °C; 1,085 °F). It is black or brownish-black with a metallic luster, has a Mohs hardness of 5–6 and a black streak. Magnetite is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, and this was how ancient people first noticed the property of magnetism. Lodestones were used as an early form of magnetic compass. Magnetite typically carries the dominant magnetic signature in rocks, and so it has been a critical tool in paleomagnetism, a science important in discovering and understanding plate tectonics and as historic data for magnetohydrodynamics and other scientific fields. The relationships between magnetite and other iron-rich oxide minerals such as ilmenite, hematite, and ulvospinel have been much studied, as the complicated reactions between these minerals and oxygen influence how and when magnetite preserves records of the Earth‘s magnetic field. Magnetite has been very important in understanding the conditions under which rocks form. Magnetite reacts with oxygen to produce hematite, and the mineral pair forms a buffer that can control oxygen fugacity. Commonly, igneous rocks contain grains of two solid solutions, one between magnetite and ulvospinel and the other between ilmenite and hematite. Compositions of the mineral pairs are used to calculate how oxidizing was the magma (i.e., the oxygen fugacity of the magma): a range of oxidizing conditions are found in magmas and the oxidation state helps to determine how the magmas might evolve by fractional crystallization. Small grains of magnetite occur in almost all igneous and metamorphic rocks. Magnetite also occurs in many sedimentary rocks, including banded iron formations. In many igneous rocks, magnetite-rich and ilmenite-rich grains occur that precipitated together from magma. Magnetite also is produced from peridotites and dunites by serpentinization. Magnetite is sometimes found in large quantities in beach sand. Such black sands (mineral sands or iron sands) are found in various places, such as California and the west coast of New Zealand. The magnetite is carried to the beach via rivers from erosion and is concentrated via wave action and currents. Huge deposits have been found in banded iron formations. These sedimentary rocks have been used to infer changes in the oxygen content of the atmosphere of the Earth. Large deposits of magnetite are also found in the Atacama region of Chile, Valentines region of Uruguay, Kiruna, Sweden, the Pilbara, Midwest and Northern Goldfields regions in Western Australia, New South Wales in the Tallawang Region, and in the Adirondack region of New York in the United States. Deposits are also found in Norway, Germany, Italy, Switzerland, South Africa, India, Mexico, and in Oregon, New Jersey, Pennsylvania, North Carolina, Virginia, New Mexico, Utah, and Colorado in the United States. In 2005, an exploration company, Cardero Resources, discovered a vast deposit of magnetite-bearing sand dunes in Peru. The dune field covers 250 square kilometers (100 sq mi), with the highest dune at over 2,000 meters (6,560 ft) above the desert floor. The sand contains 10% magnetite. Crystals of magnetite have been found in some bacteria (e.g., Magnetospirillum magnetotacticum) and in the brains of bees, termites, fish, some birds (e.g., the pigeon) and humans. These crystals are thought to be involved in magnetoreception, the ability to sense the polarity or the inclination of the Earth‘s magnetic field, and to be involved in navigation. Also, chitons have teeth made of magnetite on their radula, making them unique among animals. This means they have an exceptionally abrasive tongue with which to scrape food from rocks. The study of biomagnetism began with the discoveries of Caltech paleoecologist Heinz Lowenstam in the 1960s. Because of its stability at high temperatures, it is used for coating industrial water tube steam boilers. The magnetite layer is formed after a chemical treatment (e.g. by using hydrazine). Magnetite is also used as a catalyst for various industrial chemical processes, such as: Fischer-Tropsch process, the Haber-Bosch process and the water gas shift reaction. Text Source: Wikipedia; Picture: magnetite on chalcopyrite (gold-colored); Size: 7 x 6 x 4 cm; Locality: Aggeneys, Nordkap, South-Africa; Source: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0, www.mindat.org/photo-251632.html
Hematite/Specularite: Fe2O3

Hematite, also spelled as haematite, is the mineral form of iron(III) oxide, one of several iron oxides. Hematite crystallizes in the rhombohedral system, and it has the same crystal structure as ilmenite and corundum. Hematite and ilmenite form a complete solid solution at temperatures above 950 °C. Hematite is a mineral, colored black to steel or silver-gray, brown to reddish brown, or red. It is mined as the main ore of iron. Varieties include kidney ore, martite (pseudomorphs after magnetite), iron rose and specularite (specular hematite). While the forms of hematite vary, they all have a rust-red streak. Hematite is harder than pure iron, but much more brittle. Maghemite is a hematite- and magnetite-related oxide mineral. Huge deposits of hematite are found in banded iron formations. Grey hematite is typically found in places where there has been standing water or mineral hot springs, such as those in Yellowstone National Park in the United States. The mineral can precipitate out of water and collect in layers at the bottom of a lake, spring, or other standing water. Hematite can also occur without water, however, usually as the result of volcanic activity. Clay-sized hematite crystals can also occur as a secondary mineral formed by weathering processes in soil, and along with other iron oxides or oxyhydroxides such as goethite, is responsible for the red color of many tropical, ancient, or otherwise highly weathered soils. The name hematite is derived from the Greek word for blood (haima), because hematite can be red, as in rouge, a powdered form of hematite. The color of hematite lends it well in use as a pigment. The English name of the stone is derived from Middle French: Hématite Pierre, which was imported from Latin: Lapis Hæmatites, which originated from Ancient Greek (haimatit lithos, “blood-red stone”). Ochre is a clay that is colored by varying amounts of hematite, varying between 20% and 70%. Red ochre contains unhydrated hematite, whereas yellow ochre contains hydrated hematite (Fe2O3 • H2O). The principal use of ochre is for tinting with a permanent color. The red chalk winning of this mineral was one of the earliest in history of mankind. The powdery mineral was first used 164,000 years ago by the Pinnacle-Point man obviously for social differentiation. Hematite residues are also found in old graveyards from 80,000 years ago. Near Rydno in Poland and Lovas in Hungary, palaeolitic red chalk mines have been found that are from 5000 BC, belonging to the Linear Pottery culture at the Upper Rhine. Rich deposits of hematite have been found on the island of Elba that have been mined since the time of the Etruscans. Hematite‘s popularity in jewelry was at its highest in Europe during the Victorian era, and has since seen a strong resurgence in North America, especially in the western United States. Certain types of hematite or iron oxide rich clay, especially Armenian bole has been used in gilding. Hematite is also used in art such as intaglio engraved gems. Hematine is a synthetic material sold as magnetic hematite. Hematite is an antiferromagnetic material below the Morin transition at 250 K, and a canted antiferromagnet or weakly ferromagnetic above the Morin transition and below its Néel temperature at 948 K, above which it is paramagnetic. The magnetic structure of a-hematite was the subject of considerable discussion and debate in the 1950s because it appeared to be ferromagnetic with a Curie temperature of around 1000 K, but with an extremely tiny moment (0.002 ?B). Adding to the surprise was a transition with a decrease in temperature at around 260 K to a phase with no net magnetic moment. It was shown that the system is essentially antiferromagnetic, but that the low symmetry of the cation sites allows spin–orbit coupling to cause canting of the moments when they are in the plane perpendicular to the c axis. The disappearance of the moment with a decrease in temperature at 260 K is caused by a change in the anisotropy which causes the moments to align along the c axis. In this configuration, spin canting does not reduce the energy. Hematite is part of a complex solid solution oxyhydroxide system having various degrees of water, hydroxyl group, and vacancy substitutions that affect the mineral‘s magnetic and crystal chemical properties. Two other end-members are referred to as protohematite and hydrohematite. Hematite is present in the waste tailings of iron mines. A recently developed process, magnetation, uses huge magnets to glean waste hematite from old mine tailings in Minnesota‘s vast Mesabi Range iron district. The spectral signature of hematite was seen on the planet Mars by the infrared spectrometer on the NASA Mars Global Surveyor („MGS“) and 2001 Mars Odyssey spacecraft in orbit around Mars. The mineral was seen in abundance at two sites on the planet, the Terra Meridiani site, near the Martian equator at 0° longitude, and the second site Aram Chaos near the Valles Marineris.Several other sites also showed hematite, e.g., Aureum Chaos. Because terrestrial hematite is typically a mineral formed in aqueous environments, or by aqueous alteration, this detection was scientifically interesting enough that the second of the two Mars Exploration Rovers was targeted to a site in the Terra Meridiani region designated Meridiani Planum. In-situ investigations by the Opportunity rover showed a significant amount of hematite, much of it in the form of small spherules that were informally named „blueberries“ by the science team. Analysis indicates that these spherules are apparently concretions formed from a water solution. „Knowing just how the hematite on Mars was formed will help us characterize the past environment and determine whether that environment was favorable for life,“ .. „One big question, of course, is whether life ever started on Mars. This mission probably won‘t tell us that, but it may well lead to future mission that can answer that question.“ Text Source: Wikipedia; Picture Source: „Lysippos“.
